Custom Service
- Overview
- Polyclonal Antibody
- Monoclonal Antibody
- Epitope Design & Peptide Synthesis
- IHC Services
- Gene Synthesis
- Recombinant Protein Production
- Stable Cell Lines Development
- In vitro & in vivo Biological Assays
- Bioinformatics Services
- Custom Recombinant Antibody Development
- Fast-Track Monoclonal Antibody Production Service
Custom Monoclonal Antibody Development/Production Service in India
Customers interested in custom monoclonal antibody development/production projects with us, can opt for any one of the below mentioned custom monoclonal antibody development/production services available with us. A. Partial Custom Monoclonal Antibody Development/Production ServiceB. Complete Custom Monoclonal Antibody Development/Production Service-Peptide For custom monoclonal antibody development/production service from peptide, customer provides the sequence information of the protein (Ref. The Gene Accession number from NCBI gives details of antigen information). We go via the peptide route to produce antibody for you. This is achieved by selecting the epitopic region (possible immunogenic sites) from the sequence and immunizing the mice with the peptide conjugated to a carrier molecule. C. Complete Package for Custom Monoclonal Antibody Development/Production Service-Partial recombinant protein For custom monoclonal antibody development service from partial recombinat protein, customer provides the sequence information of the protein (Ref. The Gene Accession number from NCBI gives details of antigen information). Custom monoclonal antibody development service includes all the features of partial package for custom polyclonal service apart from the following. 1. Identification of target antigen 2. Gene Synthesis and Partial Recombinant protein purification (Upto 200 amino acid) N.B. The centrality of custom monoclonal antibody development/production service phases remains same except that antigen is provided by us in the complete packages. To know the details of pricing for peptide design and synthesis please see details of our custom peptide synthesis.
Start up
There are four phases for each custom monoclonal antibody development project. Each phase has its own duration and pricing structure. Movement from one phase to the other would be discussed with the customer and depending on the progress, decision would be taken. We shall do our best to get things done faster. However, there are multiple factors those influence the progress while meeting the dead line and that has to be sorted out amicably. Customer can start a project for a particular phase with a single purchase order (PO) and move on to the next phase with a separate PO or send order including all the features at a time.
We emphasize open communication between researchers and our Scientists/Product Manager at the out set of each custom monoclonal antibody development project. In the technical brochure, attempt has been made to give as much information on custom monoclonal anitbody development service as we can but still there may be points which should be clarified before start of each project. Routine e-mail and discussion is encouraged.
Here are some more basics before we detail the project flow:
Phase I: Antisera Development in Mouse 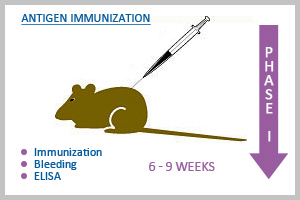
Choice of animal is Balb/c mice. We introduce 4 mice for each antigen. Quantity of immunogen required is minimum 5 mg. We prefer the customer to supply it in a single batch. However, it can also be accepted in two batches if the protein is difficult to express or purify. We have a standard immunization schedule specific for peptide antigen and for native protein/recombinant protein antigen. However, customer can feel free to advise us the immunization schedule depending on the antigen they supply. At the end of immunization the animals are bled and checked for the specific titer by an indirect ELISA using free immunogen as antigen and if desired, Western blot can also be done. At the end of this stage, we would send at least 20 µl of test sera sample along with the pre immune sera to the customer for testing at their end. ABGENEX gives guarantee of ELISA positive sera only on antigen supplied by us. For customer supplied antigen, we can attempt a few more boosters if the sera are negative or non specific in the test system, before calling it off. After results are available and acceptable to the customer, we would proceed to Phase II.
Phase II: Fusion
Phase III: Sub-cloning and isotyping
Phase-IV: Large scale production of mAb and Purification
Important Notes:
Time duration given above does not include the time taken by the customer for validating test bleeds or hybridoma supernatants provided and sending us the test results. Moreover, time duration might extend at every stage in case the customer has trouble in analyzing the samples sent by us and requests our help to sort the issue. Also, time duration might prolong if the customer requests multiple shipments other than those described above. Customers are advised to clarify with our technical service before submission of the PO. While placing the PO, kindly ensure to write the In-voice number, Cat No., Additional Services solicited, mode of payment, name and phone number of the organization. Always ask for Immunogen Form for each of our antibody production. Please e-mail us if you want any further clarification.
Additional Services
Terms and Conditions:
|

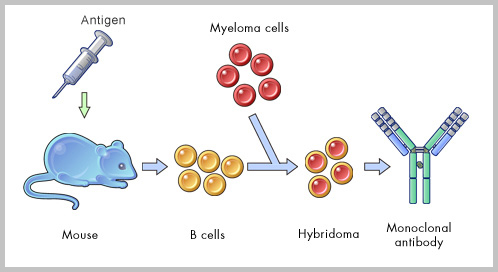 ABGENEX develops and commercializes novel reagents suitable for research in human biology in general and disease processes in particular in India. We are a leading custom monoclonal antibody developing company in India. Availability of newer reagents paves the way for development of new diagnostic assays and helps in finding potential therapies for such diseases. We possess the capacity to develop more than 400 polyclonal and 100 monoclonal antibodies per year in India (with application validated in different test systems) through our in-house R&D as well as from scientific collaborations in India and abroad.
ABGENEX develops and commercializes novel reagents suitable for research in human biology in general and disease processes in particular in India. We are a leading custom monoclonal antibody developing company in India. Availability of newer reagents paves the way for development of new diagnostic assays and helps in finding potential therapies for such diseases. We possess the capacity to develop more than 400 polyclonal and 100 monoclonal antibodies per year in India (with application validated in different test systems) through our in-house R&D as well as from scientific collaborations in India and abroad. 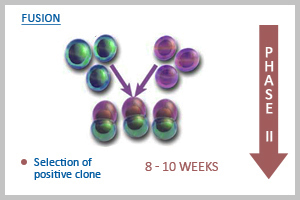 After customer checks the specificity and affinity of the antibody in mouse sera, we decide to go for generation of mAb using PEG fusion technology (The mouse to be fused would be specified by the customer). Splenocytes are fused with suitable myeloma partner, and hybridoma clones are raised. Growing colonies are screened for their Ab producing capacity and detected by ELISA and if desired western blot (depending on the availability of the testing materials) can also be done free of charge. Strong ELISA positive clones are selected, expanded and frozen. At this stage, a minimum of 5-10 ml of culture supernatant from positive clones is also supplied to customer for validating antibody in desirable application system. If the customer wants to wind up the project at this stage, then we supply hybridoma cells (ELISA positive) to customer. However, if the customer wants the project to be continued, we send everything at the completion of each project. Customer must be aware that ABGENEX can guarantee only ELISA positive clones.
After customer checks the specificity and affinity of the antibody in mouse sera, we decide to go for generation of mAb using PEG fusion technology (The mouse to be fused would be specified by the customer). Splenocytes are fused with suitable myeloma partner, and hybridoma clones are raised. Growing colonies are screened for their Ab producing capacity and detected by ELISA and if desired western blot (depending on the availability of the testing materials) can also be done free of charge. Strong ELISA positive clones are selected, expanded and frozen. At this stage, a minimum of 5-10 ml of culture supernatant from positive clones is also supplied to customer for validating antibody in desirable application system. If the customer wants to wind up the project at this stage, then we supply hybridoma cells (ELISA positive) to customer. However, if the customer wants the project to be continued, we send everything at the completion of each project. Customer must be aware that ABGENEX can guarantee only ELISA positive clones.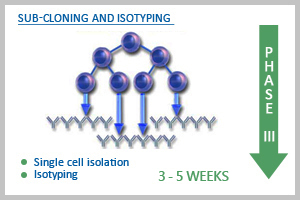 After thorough investigation when the validity of the antibody application is confirmed, repeated attempts would be made to generate stable single cell clone stage through limiting dilution. Sub-cloning would be continued until we get a stable clone that produces antibody continuously and specifically. At this stage, the culture supernatants from two sub-clones would be sent to customer for verification. We would also do the isotyping of the clones at this stage and preferably select IgG isotypes, unless the customer wants otherwise. We will send the live cells at room temperature to avoid any damage due to the frozen cells during dry ice shipment. We can validate the mAb for other applications; for example, FACS, IP, IHC and IF at extra charge (see Additional Services).
After thorough investigation when the validity of the antibody application is confirmed, repeated attempts would be made to generate stable single cell clone stage through limiting dilution. Sub-cloning would be continued until we get a stable clone that produces antibody continuously and specifically. At this stage, the culture supernatants from two sub-clones would be sent to customer for verification. We would also do the isotyping of the clones at this stage and preferably select IgG isotypes, unless the customer wants otherwise. We will send the live cells at room temperature to avoid any damage due to the frozen cells during dry ice shipment. We can validate the mAb for other applications; for example, FACS, IP, IHC and IF at extra charge (see Additional Services). We select the clone suggested by customer. We scale up the mAb production using spinner flasks in continuous culture method. Then we purify the mAb from culture supernatant using specialized matrix column. We provide the purified antibody along with QC data.
We select the clone suggested by customer. We scale up the mAb production using spinner flasks in continuous culture method. Then we purify the mAb from culture supernatant using specialized matrix column. We provide the purified antibody along with QC data.














